Kitmondo
06 Feb 2015
This article was originally published in Electrochemical and Metallurgical Industry Publication of March 1907. Information within this article is therefore correct as of 1907. The publication of this material aims to provide historical insight on the subject and its place in industry.
At a recent meeting of the English Ceramic Society, Dr. R. S. HUTTON, of the University of Manchester, presented an interesting lecture, with demonstrations, on electric heating and its application to the fusion and firing of refractory materials. The electric furnace served at first solely for the initiation of new industrial processes, demanding temperatures quite beyond the scope of fuel heating. But now electric heating is being widely applied in cases where the temperatures required are just within the limit attainable with gas or coal firing, and the general tendency is undoubtedly towards its use for still lower ranges of temperature. Dr. Hutton is careful to emphasize that there is no panacea in the word electricity, and that improvements in economy and convenience are the explanation of the extended use of the electric furnace beyond its original high-temperature sphere. As a matter of fact the success of the application of electric heating often depends more on the appalling inefficiency, due to the wasteful employment of coal, in the process being displaced than upon any intrinsic advantage of electric heating.
The chief advantages of the application of electricity for heating are:
1) That it enables the heat to be generated just where it is required - inside rather than outside the heating chamber.
2) The simplicity of a delicate regulation of temperature.
3) The ease with which the furnace or kiln can be provided with a really efficient heat insulation, capable of economizing the thermal losses to the outside air.
Dr Hutton's paper makes suggestions as to what might be hoped from the electric furnace in the pottery industry, and discusses in the first part the use of the electric furnace for experimental work in the laboratory of a pottery. The principal object would be a thorough and methodical experimental study of the changes in the physical properties of the various oxides and other bodies which enter into the composition of their raw materials, caused by a variation in the temperature at which they have previously been fired.
Something has already been done in this direction. Dr. Hutton mentions the work of A. Ditte on magnesia and H. Le Chatelier on silica. Some more recent work on magnesia, magnetic oxide of iron and alumina is interesting, as it shows how very greatly the properties of these materials are altered by exposure to high temperatures in the electric furnace. In the case of alumina at least application has already been made to the ceramic industry.
Another application for which the electric furnace is specially suitable is the determination of the temperature at which a ceramic material fuses or, perhaps, rather, the determination of the point at which the material begins to soften and lose its shape.
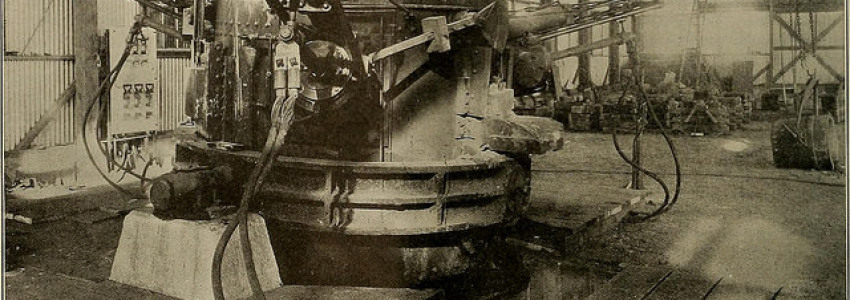
A selectric furnace suitable for laboratory work of this kind some simple types of resistance and of arc furnaces are described by the author. Among the resistance furnaces he distinguishes between such with metallic heating wires and such with carbon resistors. In the latter solid carbon, for instance, in form of carbon tubes may be used, or the carbon may be applied in granular form so as to surround the heating chamber. The granular carbon should be ground, sieved and graded; the heating being much more uniform if the grains are approximately of the same size. Furnaces of this type have been widely adopted; at the Berlin Porcelain Works they have been used up to about 1,700°.
In the second part of his paper Dr. Hutton reviews several industrial electric furnace processes. He first speaks of carborundum and siloxicon. He mentions the work going on in Niagara Falls, tending to put the manufacture of bricks, crucibles, etc. of siloxicon on a commercial basis. Much attention has also been given of late to the problem of agglomerating the powdered carborundum with a view to making strong vessels of this material. Amongst other similar uses the lining of the kilns with bricks coated with a thin layer of carborundum may be mentioned.
After a few notes on artificial graphite the author gives a review on the fusion of alumina. The object of this work, in the first place, was the production of artificial rubies. After much painstaking work, in which other French scientists have taken part, Verneuil has succeeded in producing fairly large masses, and has recently described his most ingenious method of working.
The author then referred to the very successful commercial work of the Norton Emery Co. who produce alundum, that is, artificial emery, made by fusion and purification of alumina in the electric furnace. At Rheinfelden a similar product is being manufactured, but is called "Diamantin." The Gold-schmidt Thermit Co. is also employing as an abrasive the fused alumina, which occurs as a by-product of the processes which they carry out.
In this connection is to be mentioned the application of fused alumina to the production of a new "pottery body." This development has been carried out by an important German firm of potters in conjunction with a leading chemical works. The different articles made from this new material are said to possess to a remarkable degree the power of withstanding sudden heating to a high temperature. This is due to the very small contraction which occurs in articles made with suitable mixture of the fused alumina and clay.
It would indeed be of interest to know the relative behavior of other completely "shrunk" oxides, etc., when used in a similar manner; by actual fusion in the electric furnace these materials can be obtained in a condition in which subsequent heating causes no further contraction. The strength of bodies made of such materials when subjected to severe thermal treatment probably depends much more upon the completeness of this "shrinking" than upon the thermal conductivity.
Dr. Hutton then speaks about magnesia. Since magnesia is the most refractory of the commonly occurring oxides, it is surprising that it is not more widely employed for the construction of vessels capable of withstanding high temperatures. Up to the present, however, ordinary calcined magnesia has been found none too well suited for this purpose. Magnesia bricks, crucibles, etc., of nearly all makes require very great care in handling, and it is particularly necessary in furnace work to heat them evenly and slowly, otherwise fracture occurs.
Recently the electric furnace has been used to fuse or shrink magnesia and much may be expected by the application of such products for the manufacture of crucibles, etc.
Pure magnesia tubes and other vessels of small size are now made by the Royal Porcelain Factory, at Berlin, but on account of their high cost are obviously of greater importance for scientific than - for technical work. There is, however, nothing to hinder the cheap production of electrically shrunk magnesia on a large scale, so that further developments along these lines may confidently be expected.
Arc furnaces very similar in general type to those employed in the manufacture of calcium carbide have been used technically for the production of fused alumina and magnesia, and, where it is desirable to produce a really fused and liquid product, there are somewhat great difficulties in the way of using a resistance furnace with material packed around a central core. On the other hand, much can be done with resistance furnaces, and large masses of magnesia can be heated to near the melting point, and caused to recrystallize with a very simple type of furnace, and with considerably lower power expenditure than is incurred with the arc type of furnace.
The author finally made a few remarks on what he calls silica glass, or what is called by others fused silica or fused quartz. The manufacture of this product has within the last few years shown signs of developing into a flourishing little industry.
The quartz fibers of Boys were followed by the tubes and small vessels of Shenstone, but in both cases it was the oxy-hydrogen blow-pipe which served as the source of heat. The working of the material was consequently both tedious and expensive.
Recently the electric furnace has been called into service with most satisfactory results. Relatively large tubes have been obtained from quartz crystal of Calais sand, both by indirect heating with the electric arc and also by passing the electric current through a carbon core surrounded by sand. "Recently the electrical process has been de-veloped, and a method discovered for blowing and shaping vessels from the semi-fluid material produced around an electrically-heated core. With these and other improvements the Thermal Syndicate, of Wallsend-on-Tyne, is producing large pipes, bricks, dishes, insulators, pyrometer tubes and a variety of other ceramic articles."
The advantages of fused silica are its. low coefficient of expansion, its highly refractory nature and its extreme hardness. In the discussion which followed, Dr. J. W. Mellor said that the properties of fused silica reminded him of fireproof china, "and some of us, on the morrow, will no doubt try how this substance will work in pottery bodies." He also had no hesitation in saying that the electric furnace opens up great possibilities in the preparation of "a new palette of pottery colors."
Image Credit: Internet Archive Book Images